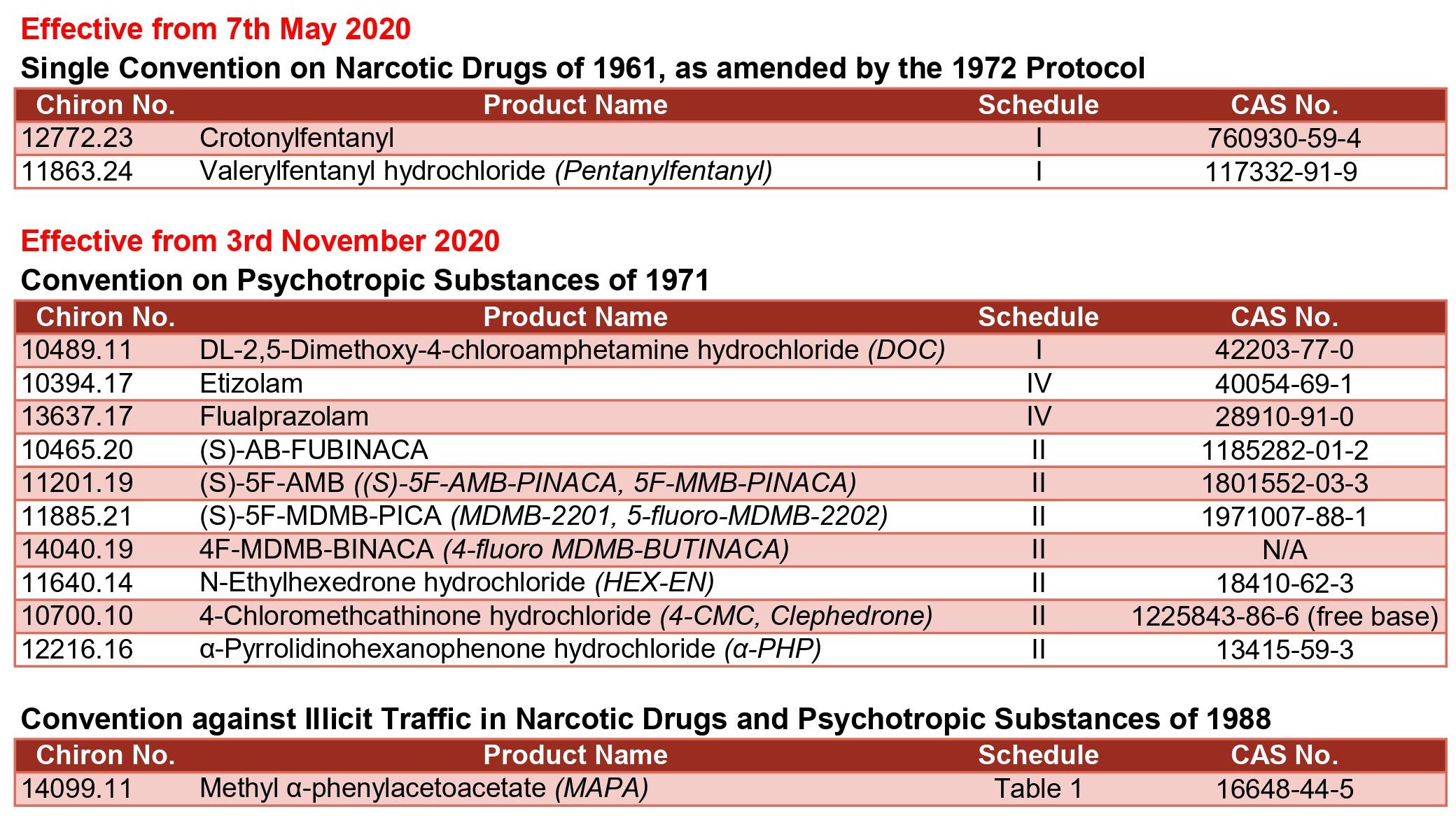
New International Drug Controls

In December 2019 The World Health Organization (WHO) recommended scheduling of 12 New Psychoactive Substances (NPS) and one precursor. The decision was adopted by the Commission on Narcotic Drugs (CND) in March 2020 and communicated to the WHO and UN Member States on 7th May. Control of two fentanyl analogues took immediate effect, whilst the remaining decisions will enter into force in November 2020.
The WHO recommended that APINACA (AKB-48) should be kept under surveillance.
What does this mean for Drug Testing Laboratories?
When a drug becomes Internationally Controlled, Member States are required to provide the International Narcotics Control Board (INCB) with an estimate of their medical and scientific requirements for the year. The status of each country's estimates is published monthly on the INCB website, and is a sign to exporting authorities that the receiving country is compliant. Delays in providing and approving estimates, not uncommon for newly scheduled compounds, can result in longer than usual delivery times for reference materials. Ordering well in advance is recommended.
View our list of available reference materials here.